G Protein-Coupled Receptor Trafficking in Health and Disease: Lessons Learned to Prepare for Therapeutic Mutant Rescue in Vivo
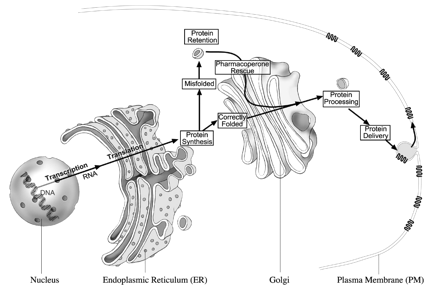 Fig. 4.
Fig. 4. Cellular sites associated with protein synthesis. Proteins are synthesized in the ER and assessed for overall quality. Folding is facilitated by interaction of the nascent polypeptide with molecular chaperones. Misfolded and misassembled products are retained in the ER and exposed to resident chaperones to attempt folding. Eventually, misfolded proteins are dislocated into the cytoplasm for proteosomal degradation after dissociation of the molecular chaperones. Alternatively, defective proteins may be exported to and retained by the Golgi apparatus, retrotranslocated to the ER where correct folding is again attempted, or diverted to lysosomes for degradation. Mature products are then exported to their final destination (the PM). Pharmacoperones can frequently rescue misfolded proteins by correcting folding and allowing them to escape retention by the QCS and route to the plasma membrane where they are able to bind ligand and couple to the effector system.
This Article
- Pharmacological Reviews September 2007 vol. 59 no. 3 225-250


Inga kommentarer:
Skicka en kommentar