A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody.
Alzheimers Res Ther. 2021 Apr 17;13(1):80. doi: 10.1186/s13195-021-00813-8.
PMID: 33865446
Free PMC article.
Clinical Trial.
BACKGROUND: Lecanemab (BAN2401), an IgG1 monoclonal
antibody, preferentially targets soluble aggregated amyloid beta
(Abeta), with activity across oligomers, protofibrils, and insoluble
fibrils. ...CSF biomarkers were supportive of a treatment effect. Lecanemab was …
Lecanemab in Early Alzheimer's Disease.
N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29.
PMID: 36449413
Clinical Trial.
Participants were randomly assigned in a 1:1 ratio to receive intravenous lecanemab (10 mg per kilogram of body weight every 2 weeks) or placebo. ...Lecanemab resulted in infusion-related reactions in 26.4% of the participants and amyloid-related imaging abnormaliti …
Lecanemab: First Approval.
Drugs. 2023 Mar;83(4):359-365. doi: 10.1007/s40265-023-01851-2.
PMID: 36856953
Review.
Lecanemab (lecanemab-irmb; LEQEMBI) is
a humanized immunoglobulin gamma 1 (IgG1) against aggregated soluble
and insoluble forms of amyloid-beta peptide. ...This article summarizes
the milestones in the development of lecanemab leading to this first approval f …
Lecanemab in patients with early Alzheimer's
disease: detailed results on biomarker, cognitive, and clinical effects
from the randomized and open-label extension of the phase 2
proof-of-concept study.
Alzheimers Res Ther. 2022 Dec 21;14(1):191. doi: 10.1186/s13195-022-01124-2.
PMID: 36544184
Free PMC article.
Clinical Trial.
The objective of this analysis is to report results from
study 201 blinded period (core), the open-label extension (OLE), and gap
period (between core and OLE) supporting the effectiveness of lecanemab. METHODS: The lecanemab study 201 core was a double-blind, rando …
Lecanemab for Alzheimer's disease.
BMJ. 2022 Dec 19;379:o3010. doi: 10.1136/bmj.o3010.
PMID: 36535691
No abstract available.
Impact of Anti-amyloid-β Monoclonal Antibodies on the
Pathology and Clinical Profile of Alzheimer's Disease: A Focus on
Aducanumab and Lecanemab.
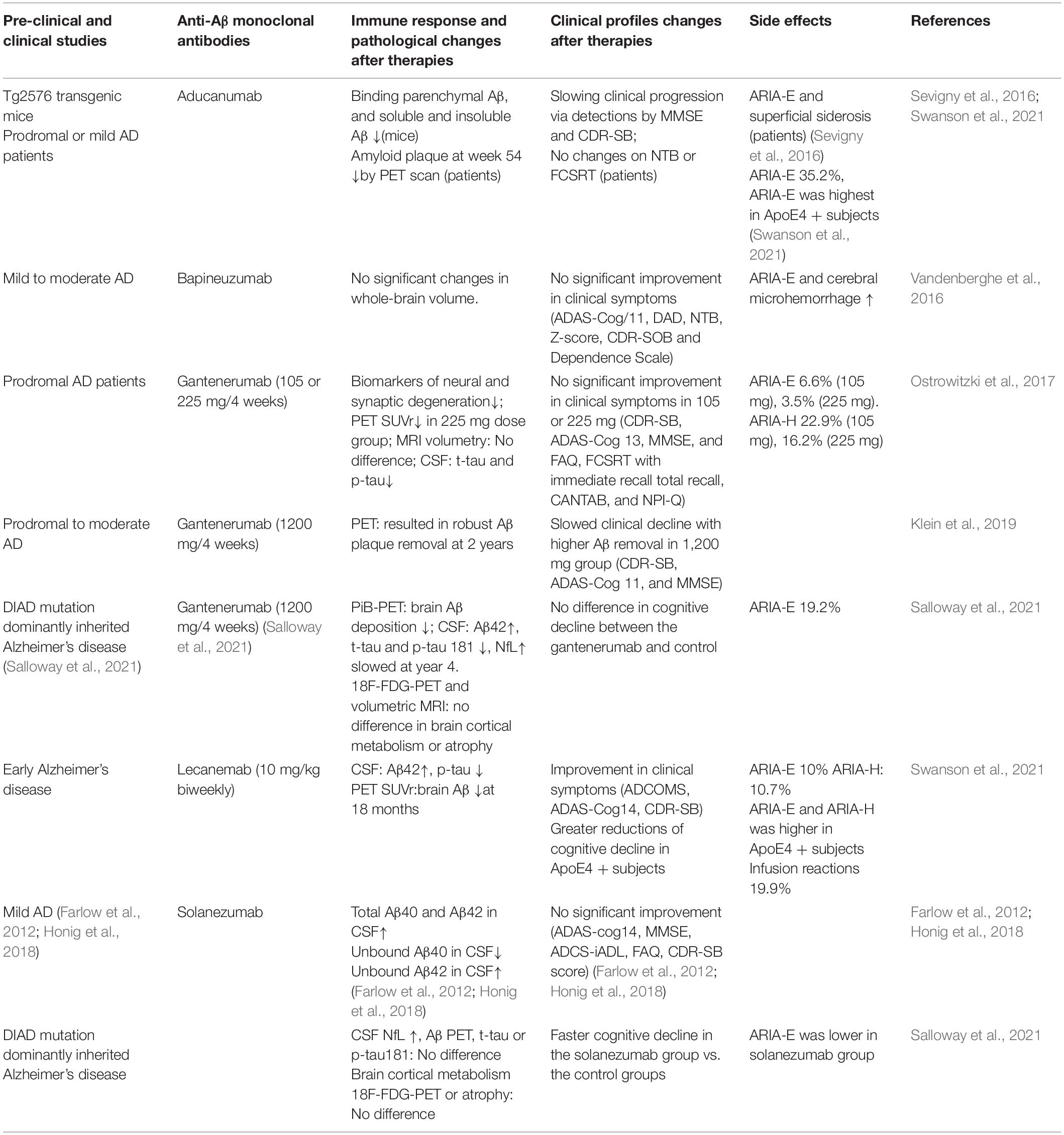
Front Aging Neurosci. 2022 Apr 12;14:870517. doi: 10.3389/fnagi.2022.870517. eCollection 2022.
PMID: 35493943
Free PMC article.
Review.
., aducanumab, bapineuzumab, gantenerumab, solanezumab, and lecanemab)
have been developed successively and conducted in clinical trials based
on the theory that a systemic failure of cell-mediated Abeta clearance
contributes to AD occurrence and progression. ...Specifical …https://www.frontiersin.org/files/Articles/870517/fnagi-14-870517-HTML/image_m/fnagi-14-870517-t001.jpg

Lecanemab for Alzheimer's disease: tempering hype and hope.
Lancet. 2022 Dec 3;400(10367):1899. doi: 10.1016/S0140-6736(22)02480-1.
PMID: 36463893
No abstract available.
Long-Term Health Outcomes of Lecanemab in Patients with Early Alzheimer's Disease Using Simulation Modeling.
Neurol Ther. 2022 Jun;11(2):863-880. doi: 10.1007/s40120-022-00350-y. Epub 2022 Apr 25.
PMID: 35469060
Free PMC article.
The mean time to mild, moderate, and severe AD dementia was longer for patients in the lecanemab
+ SoC group than for patients in the SoC group by 2.51, 3.13, and 2.34
years, respectively. ...The model also predicted a lower lifetime
probability of admission to institution …
Lecanemab, Aducanumab, and Gantenerumab - Binding
Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and
Side Effects in Clinical Trials for Alzheimer's Disease.
Neurotherapeutics. 2023 Jan;20(1):195-206. doi: 10.1007/s13311-022-01308-6. Epub 2022 Oct 17.
PMID: 36253511
Free PMC article.
All three antibodies bound monomers with low affinity. However, lecanemab and aducanumab had very weak binding to monomers, and gantenerumab somewhat stronger binding. Lecanemab was distinctive as it had tenfold stronger binding to protofibrils compared to fibrils. …
Lancet Neurol. 2023 Feb;22(2):106-108. doi: 10.1016/S1474-4422(22)00529-4.
PMID: 36681438
No abstract available.

Inga kommentarer:
Skicka en kommentar